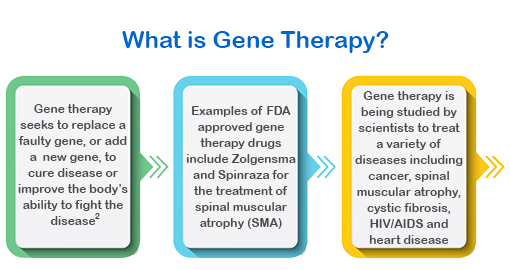
There has been a lot of discussion about gene therapy lately with the recent FDA approval of Zolgensma, the $2.1 million treatment that seeks to cure spinal muscular atrophy (SMA) in children under two years of age. Researchers are still studying ways in which gene therapy can be used to treat a wide range of diseases. Currently there are 17 cellular and gene therapy products approved by the FDA.¹
Gene therapy treatments are currently being tested for use on a variety of illnesses, in some cases to cure diseases and in others to increase the patient’s ability to fight the disease. FDA approved cellular and gene therapy products include immunotherapies and cancer vaccines. The research for these products in the United States continues to grow at a fast rate.¹ So, what exactly is gene therapy and how will these scientific advances affect the future of claims?
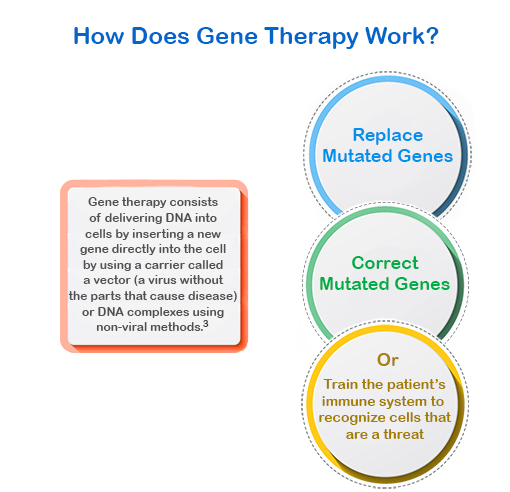
The cost of gene therapy treatments that seek to improve the quality of a patient’s life can cost up to $825,000 per year, administered yearly for the duration of the patient’s life. Other treatments that seek to cure the disease in a one-time only injection can cost up to $2.1 million or more.⁴ As more high cost treatments like gene therapy enter the market, the rate of claims in excess of $1 million will continue to increase. As these costs continue to rise, Tokio Marine HCC – Stop Loss Group (TMHCC) is closely monitoring and analyzing them to see how stop loss rates are impacted by the changing market.

TMHCC
is committed to keeping you informed and up-to-date on industry news and changes that affect your clients' self-funding needs. TMHCC is a leading provider of medical stop loss insurance provided through brokers, consultants and third-party administrators. By listening to the demands of the market, we have developed exceptional products, unparalleled resources and value-added services that set us apart in the industry.
Visit our website to learn more about our innovative stop loss, Taft-Hartley, captive and organ transplant solutions.
1. U.S. Food & Drug Administration," Approved Cellular Gene Therapy Products"
2. Mayo Clinic, "Gene Therapy"
3. The American Society of Gene & Cell Therapy
4. Data from Stacy Borans, MD, Founder and Chief Medical Officer of Advanced Medical Strategies (AMS)